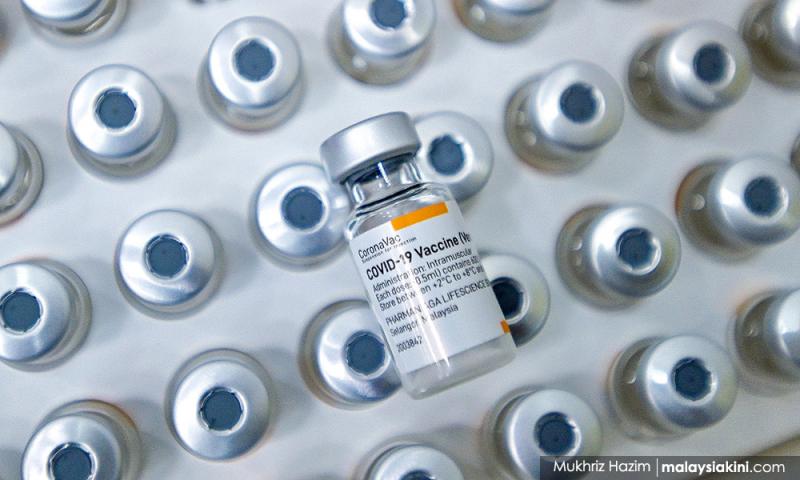
Sirim conducts various levels of testing to ensure Sinovac safety
Sirim Bhd is conducting various levels of testing to validate the process while ensuring a safe and efficacious final product of vaccine in injectable vials for Sinovac, which were received in bulk quantities from China via Pharmaniaga Bhd.
Sirim general manager for the Industrial Biotechnology Research Centre (IBRC) Dr Ahmad Hazri Ab Rashid said the tests needed are part of the process validation for collecting and evaluating data which is provided during the process design stage throughout production, to establish scientific evidence such as toxicity, stability and potency of the bulk and final product to assure safety and quality...
Verifying user
RM12.50 / month
- Unlimited access to award-winning journalism
- Comment and share your opinions on all our articles
- Gift interesting stories to your friends
- Tax deductable
 Bernama
Bernama