China's CanSino reports 'positive' interim data from Covid mRNA booster trial
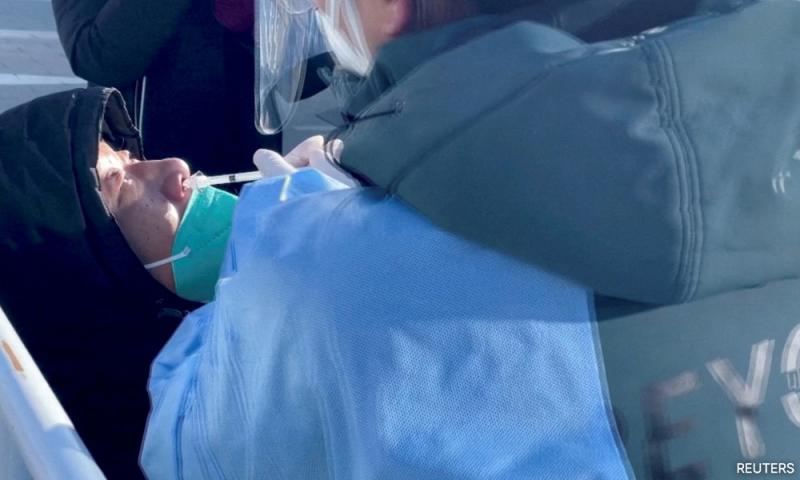
China's CanSino Biologics Inc reported yesterday "positive" interim data from its experimental Covid-19 mRNA booster vaccine in a mid-stage clinical trial, as the country races to tame a severe outbreak of infections.
Infections have spiralled since China abandoned its strict "zero Covud" policy last month after protests against tight restrictions placed on its citizens.
The CanSino booster vaccine is one of China's first home-grown potential vaccines based on mRNA technology.
The 433-person trial of those aged 18 and above tested a booster shot of the vaccine, called CS-2034, in people who had already received three doses of an inactivated vaccine, the drugmaker said in a filing to the Hong Kong stock exchange.
The new vaccine was tested against an additional shot of the inactivated vaccine, it said.
Inactivated vaccines, now used in China, contain a dead form of the pathogen, in this case, the Covid-causing virus SARS-COV-2, to help the immune system trigger a response without causing illness.
China has nine domestically-developed Covid vaccines approved for use, including inactivated vaccines, but none have been adapted to target the highly-transmissible Omicron variant and its offshoots that are currently in circulation.
In the CanSino trial, 28 days following the booster shots, adults in the CS-2034 group had virus-neutralising antibodies at levels that were 27 times as high as those in the inactivated vaccine group against the original Wuhan strain, and 23 times higher against the BA.1 Omicron variant, the company said.
According to data reported by the World Health Organization on Wednesday, China's CDC analysis showed a predominance of Omicron sublineages BA.5.2 and BF.7 among locally acquired infections.
CSPC Pharmaceutical Group Limited got approval to conduct trials for its potential mRNA shot in April last year, about the same time as CanSino.
In August, it reported a "good" safety profile for the shot called SYS6006 and said it was looking to start late-stage Phase III studies overseas and in Hong Kong. It has not released a statement since.
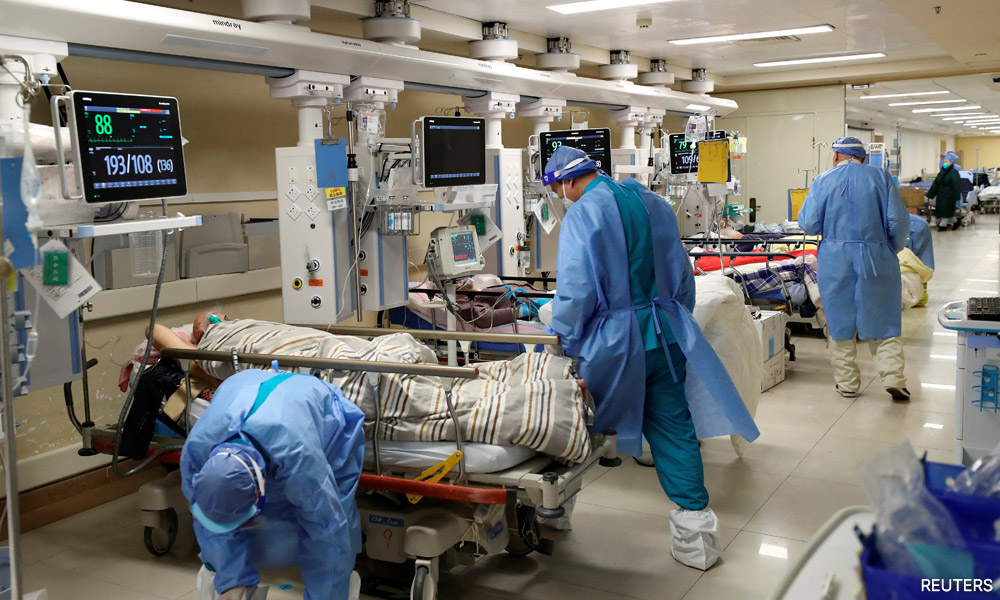
Spiralling infections are a particular concern in the world's most populous nation because the number of people who have had a booster shot is quite low, especially for the elderly.
The overall vaccination rate in the country is above 90 percent, but the rate for adults who have had booster shots drops to 57.9 percent, and to 42.3 percent for people aged 80 and older, according to Chinese government data released last month.
Overseas-developed vaccines are unavailable to the general public in mainland China, which has relied on inactivated shots by Sinopharm and Sinovac and other domestically developed options for its vaccine rollout.
While the shots are safe, some studies have suggested they are not as effective as other vaccines, such as the mRNA shots developed by Pfizer-BioNTech and Moderna.
- Reuters
RM12.50 / month
- Unlimited access to award-winning journalism
- Comment and share your opinions on all our articles
- Gift interesting stories to your friends
- Tax deductable
 Reuters
Reuters